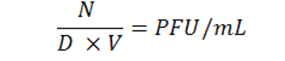Introduction
Listeria monocytogenes (L. monocytogenes) is a pathogenic bacterium that can grow at temperatures as low as 4 °C in a refrigerator [1]. Saprophytic life allows this bacterium to accommodate in non-living habitats. In addition to raw milk and soft cheese, L. monocytogenes has been found in boiled eggs and ice cream as well as fermented sausages. It has also been found in raw and cooked meat, chicken, and fish [2].
Listeria infection is triggered by many mechanisms, one of the most significant of which is the liver's growth factor, which aids in the attachment of bacteria to the adhesion factors produced by host cells. Specifically, Listeria attacks the intestinal epithelium, which attaches to host cell adhesive factors via its surface integrins and spreads across the body. This interaction triggers a particular process involving Rho-GTPases, and subsequently, bacterial endocytosis occurs in the host cell [3].
Listeria infections may result in various complications, including meningitis, sepsis, and maternal-fetal infections, with death rates of 70%, 50%, and 80%, respectively. Beta-lactam antibiotics such as penicillin and ampicillin are recommended to treat listeria infections [2, 3].
Alternative techniques for combating Listeria infection have been developed based on phage therapy. Phage P100 used to control the growth of bacteria in foods is one of the most relevant Listeria-specific phage therapies [4]. Bacteriophages are specific bacterial viruses that use host equipment to replicate and are then released by cell lysis [1]. Several bacteriophage cocktails were established to eradicate listeria contamination in fruits and meats today.
Today, the resistance of microorganisms to antibiotics has become an important and worrying issue in global health [5]. Therefore, efforts to find a suitable alternative to antibiotics that do not have side effects, especially treatment resistance, are the use of phages to treat bacterial diseases. The use of bacteriophages to remove and treat bacterial infections will not have the problems and side effects of antibiotics. Due to their specificity to the host bacterium, Bacteriophages help remove and lysis the host bacteria, and no side effects have been reported with phage therapy. There is the idea that all lytic phages are suitable for phage therapy [6]. On the other hand, one of the important applications of bacteriophages is their use in phage-based biosensors for rapid, accurate, and specific detection of pathogenic bacteria in environmental samples [7-9].
This study aimed to isolate and purify specific lytic bacteriophages of L. monocytogenes. Because, before bacteriophages are studied or their use in the field of phage therapy or biotechnology is examined, they must first be isolated and then determine their physiological characteristics as well as their host range. Contaminated environments with various bacteria such as sewage output of food processing plants are excellent sources for isolating bacteriophages, especially for isolation of the specific bacteriophages against L. monocytogenes, which is one of the most important sources of common infections in dairy foods.
Materials and Methods
Host bacteria
Lyophilized L. monocytogenes PTCC: 1783 was purchased from the Iranian Research Organization for Science and Technology (IROST) and used as a primary host to isolate specific phages.
Culture media and reagents
Trypticase soy agar (TSA) culture medium was used to isolate phage and observe phage plaques. Many phages require divalent cations such as calcium and magnesium for attachment to specific bacteria as well as intracellular growth. For this purpose, 1% (w/v) calcium chloride solution was used in these experiments [10, 11].
Collection and processing of wastewater samples
Fifty samples of polluted wastewater were collected from the river, stagnant water, and sewage sources around Khorramabad. Due to safety issues, Samples were taken with sterile syringes and collected in particular falcons. The samples were transferred to the laboratory and stored in appropriate conditions in terms of temperature. To enrich the bacteriophages in the effluent, 10 ml of the effluent was first filtered to remove the bacteria with a 45 μm injector filter into a 100 ml Erlenmeyer flask. To each effluent sample, 5% (V/V) a fresh suspension of L. monocytogenes with an optical density of 0.8 at 620 nm was added. To better bind bacteriophage to the host bacterium, 1% (w/v) calcium chloride solution was added to each effluent sample. Samples were incubated for 48 hours at 100 rpm and 30 °C [12].
Isolation of bacteriophage
To isolate specific bacteriophages, the Double Agar Overlay technique was used with some modifications. In this technique, at first, 100 µL of the L. monocytogenes with an optical density of 0.8 at 620 nm was inoculated and spread on the TSA media plate's surface containing 1.5% agar. The media plates were incubated for 3 hours at 37 °C. To prepare top agar, TSA semi-solid culture medium containing 1% agar was mixed with different amounts of treated effluent and 1% (w/v) calcium chloride solution. The top layer agar was then gently poured onto the bottom agar. The double agar overlay media plates were incubated for 24 hours at 37 °C [1, 13].
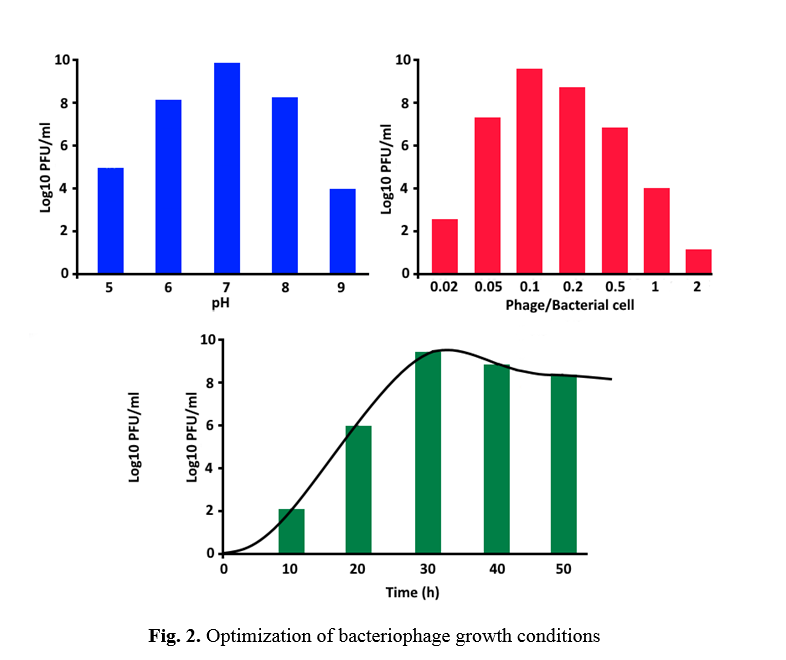
Optimization of bacteriophage growth conditions
To evaluate the ratio of phage to bacteria in the Double Agar Overlay technique, different volumes of phage (5, 10, 50, 100, 200, and 500 µL) and bacteria (50, 100, and 200 µL) were added to the culture medium. In this experimental design, by keeping the bacterial volume constant, different volume of phage were prepared to the culture medium. The experiments were performed as a factor at any time. Then the plaque formation on the culture medium was evaluated. Round and countable plaques are formed on the culture medium in a suitable ratio between phage and bacteria.
To optimize the incubation time, Double Agar Overlay plates were incubated at different times of 12, 24, 36, and 48 hours at 37 °C. Then, the plaque formation on the culture medium was evaluated.
To optimize the plaque formation temperature, the plates were incubated at different temperatures of 25, 30, 35, 37, and 40 °C. Then, the plaque formation on the culture medium was evaluated.
To optimize the plaque formation pH, phage and bacterial suspensions were cultured on media as previously mentioned as the Double Agar Overlay technique, with different pH of 5, 6, 7, 8, and 9. Then the plaque formation on the culture medium was evaluated.
Characterization of phages
To determine the host range of the isolated phage, three samples of gram-positive bacteria (Staphylococcus aureus PTCC: 1917; Bacillus cereus PTCC: 1857; L. monocytogenes PTCC: 1783) and two samples of gram-negative bacteria (Pseudomonas aeruginosa PTCC: 1690; Escherichia coli PTCC: 1769) were used.
The Double Agar Overlay technique described earlier was used to count phage plaques. Phage plaques appeared after 36 hours of incubation. Then plates containing 30 – 300 plaques were counted. Phage lysate titration was calculated using the following formula [14]:
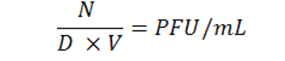
In this formula:
N = Average Number of Plaques
D = Dilution Factor
V = Volume of diluted bacteriophages added to the plate
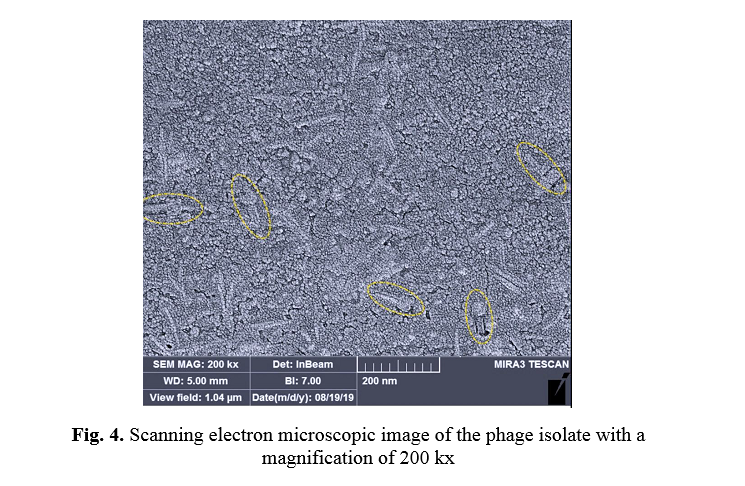
For electron microscopic observation of phage isolates, bacteriophages were first isolated from bilayer agar surface. For this purpose, after the appearance of bacteriophage plaques on the surface of Double Agar Overlay plates, 10 ml of sterilized phosphate buffered saline buffer was poured on the surface of the plates. The plates were incubated at 37 °C for 30 minutes. The agar surface was then scratched, and the supernatant was transferred into sterile microtubes and centrifuged at 8000 rpm for 15 minutes. 5 µL of supernatant was poured onto sterile slides and dried at room temperature. Finally, the FE-SEM TESCAN MIRA model of the scanning electron microscope was used to observe the morphology of the phage isolate.
Confirmation of the specificity and ability of the isolated phage to remove L. monocytogenes in milk and whey
20 ml of pasteurized milk and whey were filtered separately into a sterile falcon. 1 mL of the L. monocytogenes suspension with an optical density of 0.8 at 620 nm were added to each falcon. Each falcon was then inoculated with 0.1 ml of isolated bacteriophage. The falcons were incubated for 36 hours at 37 °C. Then 0.1 ml of each falcon were cultured on the surface of the TSA medium, and the plates were incubated again at 37 °C. After 36 hours, the bacterial growth rate was assessed and compared with the control samples that did not contain isolated bacteriophage. The present study is approved by the Ethical Committee of Yazd branch, Islamic Azad University, Yazd, Iran.
Results
Among 50 effluent samples, two contained bacteriophage, which was taken from the Khorramabad city wastewater treatment plant. Figure 1 shows an image of a Double Agar Overlay plate used to separate phage. Clear spots were the site of phage plaque formation on the culture medium. This image was related to phage isolation experiments before optimizing specific bacteriophage growth conditions.
The experimental results showed that the best ratio between L. monocytogenes and isolated bacteriophages was 10: 1, respectively. The best incubation temperature and time were 37°C and 36 hours, respectively. The best pH of the culture medium was 7. The results of the optimization tests are shown in Figure 2. Isolated phage plaques were formed as distinct transparent dots on the plate surface in these optimized conditions. Figure 3 shows the image of a Double Agar Overlay plate after optimizing the isolated bacteriophage growth conditions.

Host range determination
The results showed that the isolated bacteriophage could not lysis the Staphylococcus aureus PTCC: 1917; Bacillus cereus PTCC: 1857; Pseudomonas aeruginosa PTCC: 1690; Escherichia coli PTCC: 1769 bacteria. Phage plaques formed only on the culture medium containing L. monocytogenes. These observations proved that the isolated phage was specific to L. monocytogenes.
Titration of phage lysate
In this experiment, tube 7 contained countable phage particles, resulting in 9 × 109 pfu/ml phage lysates under optimized conditions.
Electron microscopic morphology of bacteriophage isolate
Figure 4 shows a scanning electron microscopic image of the isolated phage. As can be seen, the phage had a contractile tail and an icosahedral head, indicating this phage is in the order of Caudovirales bacteriophage.
Confirmation of the specificity and ability of the isolated phage to remove L. monocytogenes in milk and whey
This experiment added phage isolate suspension to various food samples infected with L. monocytogenes. The culture results of the infected food samples showed that the samples to which phage isolate suspension was added had a 75% reduction in the growth of L. monocytogenes compared to the control samples without bacteriophage isolate.
Discussion
Many articles have been published on the isolation of bacteriophages for therapeutic purposes [12, 15-18]. In this study, bacteriophage was isolated from the wastewater treatment plant, just as many other phages have been isolated from these sources by other researchers [19, 20]. The most important reason for choosing this type of source for the isolation of specific bacteriophages was the presence of different types of bacteria in the wastewater and, as a result, the presence of specific bacteriophages in this source.
This study optimized the most important factors affecting bacteriophage growth and phage plaque formation. The experimental results showed that the best incubation temperature and time for optimal growth of this isolated phage were 37 °C and 36 h, respectively, at pH 7. The research results by Abdelsattar et al., which investigated specific bacteriophages of Salmonella species, showed that the best incubation temperature and time for optimal growth of that isolated phage were 45 °C and 24 h, respectively at pH 7 [21]. Bigot et al. reported that the best incubation time for the specific bacteriophage of L. monocytogenes was 24 h [17].
In this study, titration of phage lysate was nine × 109 pfu/ml, which in many articles, phage lysate was between 106 and 1011 pfu/ml [13, 14]. Our results were consistent with other research results. Phage plaques formed only on the culture medium containing L. monocytogenes. These results proved that the isolated phage was specific to this bacterium. The electron microscopic morphology of isolated bacteriophage showed that the isolated phage had a contractile tail and an icosahedral head, indicating this phage is in the order of Caudovirales bacteriophage. Using this specific isolated phage in real environments showed that this phage could reduce the contamination load in the food. The elimination of L. monocytogenes growth in milk and whey samples revealed that this bacteriophage targeted bacteria and resulted in a 75% reduction in bacterial contamination.
However, eventually, to evaluate the suitability and effectiveness of the phage therapy process, a specific phage must be isolated from environmental sources, and its characteristics, such as its host range, must be determined. The following steps examine characteristics such as the absence of toxin genes and the lysogenic cycle.
Conclusion
The results suggest that phage obtained from L. monocytogenes bacterium may be used to remove or even reduce the pollution load of this pathogen in the dairy products, the agri-food, the wastes, and the other associated foods to increase food safety. This phage in food products has no side effects or toxicity on the host. In addition, phage therapy is an excellent alternative to antibiotics and reduces public health concerns about antibiotic-resistant pathogens.
Conflicts of interest
The authors declare that they have no competing interests.
Acknowledgments
The authors would like to thank the members of the biology department of the Islamic Azad University, Khorramabad Branch, Dr. Behrouz Dousti and Dr. Farhad Gilavand, for their help and cooperation.
References
[1] . Stone E, Lhomet A, Neve H, Grant IR, Campbell K, McAuliffe O. Isolation and characterization of listeria monocytogenes phage vB_LmoH_P61, a phage with biocontrol potential on different food matrices. Frontiers in Sustainable Food Systems 2020; 4(1): 1-10.
[2] . Shamloo E, Hosseini H, Moghadam AZ, Larsen HM, Haslberger A, Alebouyeh M. Importance of Listeria monocytogenes in food safety: A review of its prevalence, detection, and antibiotic resistance. Iranian Journal of Veterinary Research 2019; 20(4): 241-54.
[3]. Pushkareva VI, Ermolaeva SA. Listeria monocytogenes virulence factor Listeriolysin O favors bacterial growth in co-culture with the ciliate Tetrahymena pyriformis, causes protozoan encystment and promotes bacterial survival inside cysts. BMC Microbiology 2010; 10(1): 1-11.
[4]. Carlton RM, Noordman WH, Biswas B, De Meester ED, Loessner MJ. Bacteriophage P100 for control of Listeria monocytogenes in foods: Genome sequence, bioinformatic analyses, oral toxicity study, and application. Regulatory Toxicology and Pharmacology 2005; 43(3): 301-12.
[5] . Yahyavi Firozabadi A, Sedighi-Khavidak S, Soltan Dallal MM. The molecular epidemiology evaluation and antibiotic resistance patterns of Salmonella isolated in food outbreaks in Yazd province by culture and PCR methods. Razi Journal of Medical Sciences 2016; 23(1): 1-8.
[6] . Hyman P. Phages for phage therapy: Isolation, characterization, and host range breadth. Pharmaceuticals 2019; 12 (1): 1-23.
[7]. Singh S, Dhanjal DS, Sonali S, Thotapalli S, Kumar V, Datta S, et al. An insight in bacteriophage based biosensors with focus on their detection methods and recent advancements. Environmental Technology & Innovation 2020; 20: 101081.
[8]. Patel D, Zhou Y, Ramasamy RP. A bacteriophage-based electrochemical biosensor for detection of methicillin-resistant staphylococcus aureus. Journal of The Electrochemical Society 2021; 168(5): 57523.
[9] . Sedighi-Khavidak S, Mazloum-Ardakani M, Rabbani Khorasgani M, Emtiazi G, Hosseinzadeh L. Detection of aflD gene in contaminated pistachio with Aspergillus flavus by DNA based electrochemical biosensor. International Journal of Food Properties 2017; 20 (S1): 119-30.
[10]. Bandara N, Jo J, Ryu S, Kim KP. Bacteriophages BCP1-1 and BCP8-2 require divalent cations for efficient control of Bacillus cereus in fermented foods. Food Microbiology 2012; 31(1): 9-16.
[11]. Pham M, Mintz EA, Nguyen TH. Deposition kinetics of bacteriophage MS2 to natural organic matter: Role of divalent cations. Journal of Colloid and Interface Science 2009; 338(1): 1-9.
[12]. Shende RK, Hirpurkar SD, Sannat C, Rawat N, Pandey V. Isolation and characterization of bacteriophages with lytic activity against common bacterial pathogens. Veterinary World 2017; 10(8): 973-78.
[13] . Anderson B, Rashid MH, Carter C, Pasternack G, Rajanna C, Revazishvili T, et al. Enumeration of bacteriophage particles. Bacteriophage 2011; 1(1): 86-93.
[14] . Stephenson FH. Working with bacteriophage. Calculations for molecular biology and biotechnology, San Diego, USA: Academic Press; 2016, p. 81-96.
[15]. Urban-Chmiel R, Wernicki A, Stęgierska D, Dec M, Dudzic A, Puchalski A. Isolation and characterization of lytic properties of bacteriophages specific for M. haemolytica strains. PLoS One 2015; 10(10): 140.
[16] . Demirarslan Ö, Alasalvar H, Yildirim Z. Biocontrol of Salmonella enteritidis on chicken meat and skin using lytic SE-P3, P16, P37, and P47 bacteriophages. LWT 2021; 137: 110469.
[17] . Bigot B, Lee WJ, McIntyre L, Wilson T, Hudson JA, Billington C, et al. Control of Listeria monocytogenes growth in a ready-to-eat poultry product using a bacteriophage. Food Microbiology 2011; 28(8): 1448-452.
[18]. Zhang H, Bao H, Billington C, Hudson JA, Wang R. Isolation and lytic activity of the Listeria bacteriophage endolysin LysZ5 against Listeria monocytogenes in soya milk. Food Microbiology 2012; 31(1): 133-36.
[19]. Maal KB, Delfan AS, Salmanizadeh S. Isolation and identification of two novel escherichia coli bacteriophages and their application in wastewater treatment and coliform's phage therapy. Jundishapur Journal of Microbiology 2015; 8(3): 14945.
[20] . Beaudoin RN, Decesaro DR, Durkee DL, Barbaro SE. Isolation of a bacteriophage from sewage sludge and characterization of its bacterial host cell. River Academic Journal 2007; 3(1): 1-8.
[21] . Abdelsattar AS, Safwat A, Nofal R, Elsayed A, Makky S, El-shibiny A. Isolation and characterization of bacteriophage ZCSE6 against Salmonella spp: Phage application in milk. Biologics 2021; 1(2): 164-76.