Sat, Feb 7, 2026
[Archive]
Volume 11, Issue 3 (August 2024)
IJML 2024, 11(3): 217-230 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Owlia E, Pourrajab F. Aptamer-Based Lateral Flow Immunoassay for Detection of D-Dimer in the Blood. IJML 2024; 11 (3) :217-230
URL: http://ijml.ssu.ac.ir/article-1-545-en.html
URL: http://ijml.ssu.ac.ir/article-1-545-en.html
Reproductive Immunology Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
Full-Text [PDF 391 kb]
(374 Downloads)
| Abstract (HTML) (411 Views)
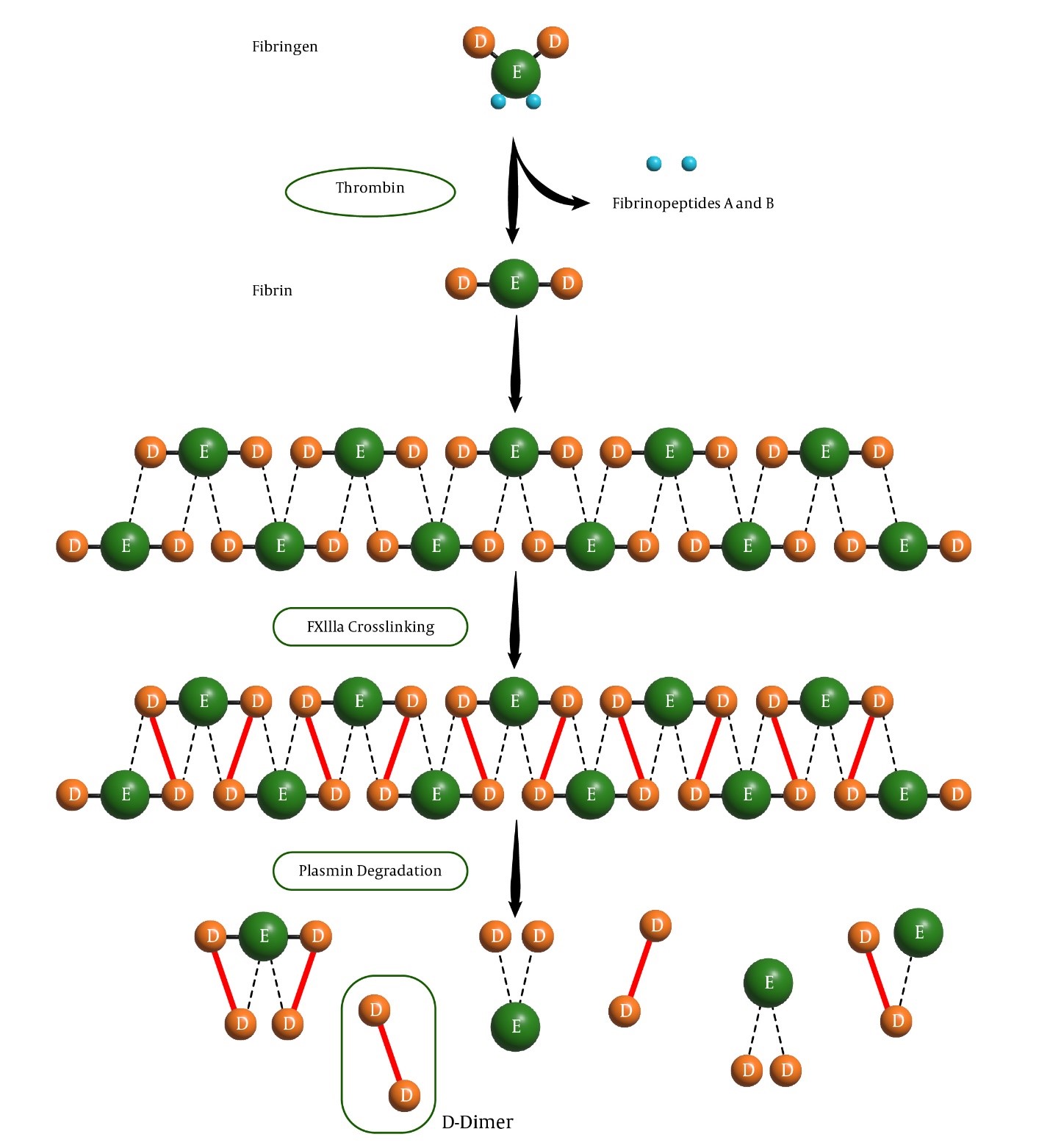
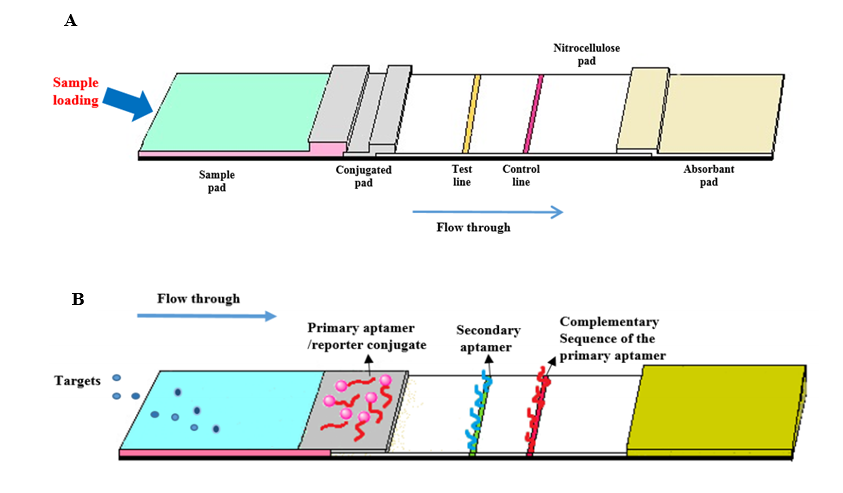
Table 4. Existing aptamer-based LFIA formats, including sandwich and competitive formats [21, 45].
Conclusion
References
Full-Text: (340 Views)
Introduction
D-dimer, a fibrin degradation product, is a well-recognized biomarker for diagnosing thrombotic disorders. The prompt and precise measurement of D-dimer levels is critical for ensuring timely clinical intervention and effective management of these conditions [1]. This literature review represents the development of an aptamer-based lateral flow immunoassay (LFIA) designed for the qualitative detection of D-dimer. The designed LFIA platform integrates the specificity of aptamer-based D-dimer recognition with the efficiency and speed of lateral flow technology, thus providing a practical point-of-care diagnostic solution for rapid detection of thrombosis. Aptamers are single-stranded DNA or RNA molecules selected for their high affinity and specificity toward target molecules [2]. They can be utilized as recognition elements in diagnostic applications [3].
Developed aptamer-based LFIAs have demonstrated excellent analytical performance, featuring a broad dynamic range and low detection limits comparable to traditional immunoassay methods [4]. Furthermore, the LFIA platform exhibited notable stability, reproducibility, and robustness, rendering it suitable for clinical applications [5].
This study underscores the effectiveness of aptamer-based LFIA as an innovative method for D-dimer detection. The approach presents several advantages, including rapid turnaround times, ease of use, and cost-effectiveness, which are crucial for point-of-care testing in resource-limited environments and emergencies. However, further optimization and validation are necessary to facilitate the translation of this technology into clinical practice, ultimately enhancing thrombosis management and patient care. The prompt and accurate measurement of D-dimer levels is crucial for diagnosing thrombotic disorders such as deep vein thrombosis, pulmonary embolism, and venous thromboembolism (VTE). Current diagnostic methods, while sensitive, are often time-consuming and require specialized equipment, limiting their use in point-of-care settings. This study aims to develop an aptamer-based LFIA for the rapid and specific detection of D-dimer, offering a cost-effective and portable solution for early diagnosis and management of thrombotic disorders [4].
D-dimer in the coagulation system
D-dimer is a degradation product of fibrin formed during fibrinolysis, the process by which blood clots are broken down [6]. Throughout coagulation activation, fibrinogen is enzymatically converted into fibrin monomers, which passively aggregate until stabilized by factor XIIIa-mediated covalent bonds between adjacent fibrin monomers, forming a stable fibrin network entrapping platelets and other blood cells [7].
The term "D-dimer" originally refers to the end products of plasmin digestion on the factor XIIIa-cross-linked fibrin clot, specifically the DD/E fragments (MW~ 190-10,000 kDa) (Fig. 1). Additionally, plasmin can degrade fibrinogen and other proteins; however, this activity is regulated by alpha-2-antiplasmin, which helps to localize fibrinolysis specifically to the site of injury, ensuring that the process is focused and effective. However, current immunoassays do not exclusively detect the DD/E complex [8] (Fig. 1).
Clearance of D-dimer fragments primarily occurs via renal excretion and reticuloendothelial system catabolism, with a plasma half-life of approximately 8 hours [9]. Unlike other coagulation markers, such as the thrombin-antithrombin complex or prothrombin fragments, D-dimer persists longer in circulation [1]. Physiologically, D-dimer is present in small amounts in healthy people and increases with age [10]. Thrombin activates factor XIII, facilitating fibrin stabilization by forming cross-linked bonds between the fibrin monomers. The breakdown of fibrin is primarily carried out by plasmin, which is generated from plasminogen through the action of tissue plasminogen activator. Plasmin cleaves fibrin into fibrin degradation products, with D-dimer being a significant byproduct resulting from the cleavage of cross-linked fibrin monomers [6]. Fibrin formation begins with the conversion of fibrinogen into fibrin monomers. This process is mediated by thrombin, which removes the A and B fibrinopeptides from the fibrinogen molecule. As a result, the fibrin monomers spontaneously polymerize into a stable polymer held together by hydrogen bonds. Selected aptamers should not react with non-cross-linked fibrinogen and detect specific epitopes on cross-linked D-dimer fragments produced from factor XIIIa-cross-linked fibrin degradation products [3].
Developed aptamer-based LFIAs have demonstrated excellent analytical performance, featuring a broad dynamic range and low detection limits comparable to traditional immunoassay methods [4]. Furthermore, the LFIA platform exhibited notable stability, reproducibility, and robustness, rendering it suitable for clinical applications [5].
This study underscores the effectiveness of aptamer-based LFIA as an innovative method for D-dimer detection. The approach presents several advantages, including rapid turnaround times, ease of use, and cost-effectiveness, which are crucial for point-of-care testing in resource-limited environments and emergencies. However, further optimization and validation are necessary to facilitate the translation of this technology into clinical practice, ultimately enhancing thrombosis management and patient care. The prompt and accurate measurement of D-dimer levels is crucial for diagnosing thrombotic disorders such as deep vein thrombosis, pulmonary embolism, and venous thromboembolism (VTE). Current diagnostic methods, while sensitive, are often time-consuming and require specialized equipment, limiting their use in point-of-care settings. This study aims to develop an aptamer-based LFIA for the rapid and specific detection of D-dimer, offering a cost-effective and portable solution for early diagnosis and management of thrombotic disorders [4].
D-dimer in the coagulation system
D-dimer is a degradation product of fibrin formed during fibrinolysis, the process by which blood clots are broken down [6]. Throughout coagulation activation, fibrinogen is enzymatically converted into fibrin monomers, which passively aggregate until stabilized by factor XIIIa-mediated covalent bonds between adjacent fibrin monomers, forming a stable fibrin network entrapping platelets and other blood cells [7].
The term "D-dimer" originally refers to the end products of plasmin digestion on the factor XIIIa-cross-linked fibrin clot, specifically the DD/E fragments (MW~ 190-10,000 kDa) (Fig. 1). Additionally, plasmin can degrade fibrinogen and other proteins; however, this activity is regulated by alpha-2-antiplasmin, which helps to localize fibrinolysis specifically to the site of injury, ensuring that the process is focused and effective. However, current immunoassays do not exclusively detect the DD/E complex [8] (Fig. 1).
Clearance of D-dimer fragments primarily occurs via renal excretion and reticuloendothelial system catabolism, with a plasma half-life of approximately 8 hours [9]. Unlike other coagulation markers, such as the thrombin-antithrombin complex or prothrombin fragments, D-dimer persists longer in circulation [1]. Physiologically, D-dimer is present in small amounts in healthy people and increases with age [10]. Thrombin activates factor XIII, facilitating fibrin stabilization by forming cross-linked bonds between the fibrin monomers. The breakdown of fibrin is primarily carried out by plasmin, which is generated from plasminogen through the action of tissue plasminogen activator. Plasmin cleaves fibrin into fibrin degradation products, with D-dimer being a significant byproduct resulting from the cleavage of cross-linked fibrin monomers [6]. Fibrin formation begins with the conversion of fibrinogen into fibrin monomers. This process is mediated by thrombin, which removes the A and B fibrinopeptides from the fibrinogen molecule. As a result, the fibrin monomers spontaneously polymerize into a stable polymer held together by hydrogen bonds. Selected aptamers should not react with non-cross-linked fibrinogen and detect specific epitopes on cross-linked D-dimer fragments produced from factor XIIIa-cross-linked fibrin degradation products [3].

Fig. 1. Fibrinogen conversion to fibrin, fibrinolysis by plasmin, and release of D-dimer, a well-known marker for thrombosis assessment.
The role of D-dimer in diagnostics
Elevated levels of D-dimer in the blood have become a key biomarker for diagnosing thrombotic disorders, such as deep vein thrombosis, pulmonary embolism, and VTE. These conditions are associated with a significant morbidity and mortality rate, making early detection and intervention crucial for improving patient outcomes [8].
D-dimer testing is a pivotal aspect of diagnosing PE, a condition characterized by the obstruction of pulmonary artery blood flow by an embolus, typically originating from lower limb vein thrombi [11].
D-dimer assays are widely utilized to rule out VTE in patients. Elevated D-dimer levels are observed not only in VTE cases but also in various conditions such as infection, pregnancy, trauma, cancer, ageing, hematomas, or interstitial haemorrhages. The quantification and qualification of D-dimer in serum are carried out using traditional methods currently available [12].
Different types of D-dimer assay
D-dimer testing is widely used in clinical practice because it can help rule out the presence of a thrombotic event, particularly when coupled with clinical risk assessment models[13]. However, despite its utility, current methods of measuring D-dimer levels have limitations, especially in point-of-care settings, where speed, cost, and ease of use are critical factors [10].
Currently, the most common diagnostic methods for measuring D-dimer levels include enzyme-linked immunosorbent assays (ELISA), immunoturbidimetric assays, and latex agglutination tests. These methods are typically highly sensitive and accurate but have several drawbacks that limit their use, especially in emergency or outpatient settings.
ELISA: While ELISA offers high sensitivity, it is a laboratory-based technique that requires sophisticated equipment, trained personnel, and a relatively long turnaround time. These factors make rapid, on-site testing impractical, particularly in emergency care or rural settings.
Immunoturbidimetric assays: These assays offer more rapid results than ELISA but are also laboratory-based and may not be as portable or easy to use in a clinical setting without proper infrastructure.
Latex agglutination tests: These provide quicker results than ELISA and immunoturbidimetric assays but often lack the sensitivity and specificity required for accurate diagnosis in all patient populations [14, 15].
Among these assays, the classical latex agglutination test and the Red cell agglutination test (SimpliRED) demonstrate the lowest sensitivity, providing positive and negative results. Despite these advancements, there is still a significant need for quick, reliable, portable, and cost-effective point-of-care diagnostics. Complex procedures often hinder existing methods, the need for expensive equipment, or limitations in sensitivity, particularly in cases where D-dimer levels are low or high but not conclusive.
Here, the aptamer-based LFIA optimized for specific detection of D-dimer would emerge as the most sensitive method for qualitative measurements. Recent developments in aptamer-based technologies offer a promising solution to address the limitations of current D-dimer assays [16].
According to the literature, adjusting the aptamer and understanding its dissociation constant (Kd) value can enable accurate detection and quantitation of D-dimer levels in the specified range.
Adjusting the Aptamer sensor amount based on D-dimer levels and Kd value is crucial for accurate detection. The Kd measures the affinity between the aptamer and its target, with lower Kd values indicating higher affinity. In this study, the Kd value was optimized to ensure accurate detection of D-dimer within the clinically relevant range of 200-1000 ng/mL. The D-dimer assay's ideal range spans 200-1000 ng/mL plasma [17].
Aptamers in immunoassays
Aptamers are single-stranded nucleic acid molecules (DNA or RNA) that can bind selectively and with high affinity to specific target molecules, including proteins like D-dimer [18]. Unlike traditional antibodies, aptamers offer advantages such as greater stability, easier synthesis, and lower cost, making them ideal candidates for diagnostic applications, particularly in point-of-care testing [19]. Aptamers, derived through the SELEX (Systematic Evolution of Ligands by Exponential Enrichment) process, are single-stranded nucleic acids, including DNA, RNA, or modified nucleic acids [20]. Since their discovery in 1990, numerous aptamers have been identified for diverse targets, encompassing metal ions, organic molecules, peptide proteins, and whole cells [21]. In contrast to antibodies, aptamers exhibit sustained high binding affinities across a broad spectrum of conditions, coupled with advantageous attributes such as facile synthesis, versatile design capabilities, and enhanced stability. Nonetheless, conventional aptasensors often necessitate specialized personnel and expensive equipment and are primarily confined to laboratory settings, thereby constraining their applicability in point-of-need and point-of-care scenarios [17].
In recent years, lateral flow aptasensors (LFAs) have emerged as a promising avenue, eliciting escalating interest due to their cost-effectiveness and enhanced adaptability compared to antibody-based systems [23]. LFAs have been extensively explored for detecting various targets, spanning toxins, proteins, and cancer cells [4].
Advantages of aptamers over antibodies in lateral flow immunoassay
The LFIA is a widely used, simple, and cost-effective diagnostic method successfully applied in numerous medical tests, such as pregnancy tests and rapid infectious disease detection. By integrating aptamers into the LFIA format, we can create a rapid, specific, cost-effective D-dimer test suitable for use in low-resource and emergency settings. The proposed aptamer-based LFIA offers the potential for enhanced sensitivity, selectivity, and speed compared to existing antibody-based assays [22].
Therefore, this study aims to optimize an aptamer-based LFIA platform for the rapid detection of D-dimer in clinical samples, thereby offering a novel solution for the early detection of thrombotic disorders at the point of care. Combining the specificity of aptamers with the speed and ease of lateral flow technology could overcome the limitations of D-dimer testing, ultimately improving patient care through more timely and accurate diagnostics [4].
Antibodies may struggle to differentiate between closely related targets like small molecules, whereas aptamers can excel in this area. For example, Jenison et al. (1994) created an RNA aptamer that could distinguish between theophylline and caffeine despite their subtle chemical similarities. Unlike immunoassays, the aptamer showed a remarkable 10,000-fold preference for theophylline over caffeine [23].
In research conducted for the World Anti-Doping Agency (WADA), a series of DNA aptamers were engineered to be capable of discriminating between the virtually identical natural pituitary human growth hormone (hGH) and recombinant hGH produced in Escherichia coli bacteria- a feat challenging with antibodies. This discrimination was feasible due to alterations in up to 2% of recombinant hGH proteins induced by the bacterial host, validated by mass spectrometry [24, 25]. Similarly, the author successfully discriminated between isoleucine (I) and threonine (T) variants of prostate-specific antigen (PSA) at position 179, a task current antibodies struggle with, employing a diaminopurine (DAP)-modified aptamer and leveraging insights from a molecular docking model. The DAP-modified aptamer exhibited approximately a 20% disparity in colorimetric absorbance signals in an ELISA-like setting between the I- and T-PSA variants [25, 26].
An often overlooked yet significant distinction between aptamers and antibodies is their smaller size and weight than common IgG antibodies. Aptamers, typically comprising 70–200 bases, generally weigh approximately 20–60 kD, contrasting with IgG antibodies weighing over 150 kD. Moreover, the size of well-known aptamers, such as the thrombin aptamer, is notably smaller at approximately 21×25 Å compared to the substantially larger IgG antibodies at 122 × 139 Å [19].
Moreover, unlike antibodies, aptamers offer a unique detection modality involving hybridization or dehybridization of polymer strands, further augmenting their versatility and potential advantages in specific applications [17]. Herein, selected aptamers are derived through the SELEX process, single-stranded DNA or modified nucleic acids [27]. Numerous aptamers have been identified for diverse targets, encompassing metal ions, organic molecules, peptide proteins, and whole cells [28]. In contrast to antibodies, aptamers exhibit sustained high binding affinities across a broad spectrum of conditions, coupled with advantageous attributes such as facile synthesis, versatile design capabilities, and enhanced stability [18, 26]. Nonetheless, conventional aptasensors often necessitate specialized personnel and expensive equipment and are primarily confined to laboratory settings, thereby constraining their applicability in point-of-need and point-of-care scenarios [27].
In recent years, aptamer-based LFIAs (Fig. 2) (Table 1) have emerged as a promising avenue, eliciting escalating interest due to their cost-effectiveness and enhanced adaptability compared to antibody-based systems [29, 30].
LFAs in sensing technology
LFAs are a well-established sensing technology known for their rapid response, easy storage, user-friendly design, and on-site applicability without requiring instrumentation or additional chemicals. They can detect targets, including mycotoxins, small molecules, heavy metals, bacteria, viruses, and proteins [31].
LFAs utilize various detector reagents, from colloidal gold to dye-containing liposomes, and recent advancements include multiplex LFAs for the simultaneous detection of multiple targets [5]. Aptamer-based LFAs utilize aptamers' specificity, which is established during the selection process. They use reporter labels like gold nanoparticles, latex spheres, and quantum dots for assay specificity. LFAs offer simple, cost-effective detection of targets with high sensitivity [29]. In aptamer-based LFIAs, the sample with the analyte moves from the sample pad to the conjugate pad and binds to a labelled aptamer. The complex travels to the nitrocellulose membrane, where an immobilized aptamer captures it. Excess unbound complex is captured on the control line. Detection creates a signal on the test line and indicates proper liquid flow on the control line [30].
Elevated levels of D-dimer in the blood have become a key biomarker for diagnosing thrombotic disorders, such as deep vein thrombosis, pulmonary embolism, and VTE. These conditions are associated with a significant morbidity and mortality rate, making early detection and intervention crucial for improving patient outcomes [8].
D-dimer testing is a pivotal aspect of diagnosing PE, a condition characterized by the obstruction of pulmonary artery blood flow by an embolus, typically originating from lower limb vein thrombi [11].
D-dimer assays are widely utilized to rule out VTE in patients. Elevated D-dimer levels are observed not only in VTE cases but also in various conditions such as infection, pregnancy, trauma, cancer, ageing, hematomas, or interstitial haemorrhages. The quantification and qualification of D-dimer in serum are carried out using traditional methods currently available [12].
Different types of D-dimer assay
D-dimer testing is widely used in clinical practice because it can help rule out the presence of a thrombotic event, particularly when coupled with clinical risk assessment models[13]. However, despite its utility, current methods of measuring D-dimer levels have limitations, especially in point-of-care settings, where speed, cost, and ease of use are critical factors [10].
Currently, the most common diagnostic methods for measuring D-dimer levels include enzyme-linked immunosorbent assays (ELISA), immunoturbidimetric assays, and latex agglutination tests. These methods are typically highly sensitive and accurate but have several drawbacks that limit their use, especially in emergency or outpatient settings.
ELISA: While ELISA offers high sensitivity, it is a laboratory-based technique that requires sophisticated equipment, trained personnel, and a relatively long turnaround time. These factors make rapid, on-site testing impractical, particularly in emergency care or rural settings.
Immunoturbidimetric assays: These assays offer more rapid results than ELISA but are also laboratory-based and may not be as portable or easy to use in a clinical setting without proper infrastructure.
Latex agglutination tests: These provide quicker results than ELISA and immunoturbidimetric assays but often lack the sensitivity and specificity required for accurate diagnosis in all patient populations [14, 15].
Among these assays, the classical latex agglutination test and the Red cell agglutination test (SimpliRED) demonstrate the lowest sensitivity, providing positive and negative results. Despite these advancements, there is still a significant need for quick, reliable, portable, and cost-effective point-of-care diagnostics. Complex procedures often hinder existing methods, the need for expensive equipment, or limitations in sensitivity, particularly in cases where D-dimer levels are low or high but not conclusive.
Here, the aptamer-based LFIA optimized for specific detection of D-dimer would emerge as the most sensitive method for qualitative measurements. Recent developments in aptamer-based technologies offer a promising solution to address the limitations of current D-dimer assays [16].
According to the literature, adjusting the aptamer and understanding its dissociation constant (Kd) value can enable accurate detection and quantitation of D-dimer levels in the specified range.
Adjusting the Aptamer sensor amount based on D-dimer levels and Kd value is crucial for accurate detection. The Kd measures the affinity between the aptamer and its target, with lower Kd values indicating higher affinity. In this study, the Kd value was optimized to ensure accurate detection of D-dimer within the clinically relevant range of 200-1000 ng/mL. The D-dimer assay's ideal range spans 200-1000 ng/mL plasma [17].
Aptamers in immunoassays
Aptamers are single-stranded nucleic acid molecules (DNA or RNA) that can bind selectively and with high affinity to specific target molecules, including proteins like D-dimer [18]. Unlike traditional antibodies, aptamers offer advantages such as greater stability, easier synthesis, and lower cost, making them ideal candidates for diagnostic applications, particularly in point-of-care testing [19]. Aptamers, derived through the SELEX (Systematic Evolution of Ligands by Exponential Enrichment) process, are single-stranded nucleic acids, including DNA, RNA, or modified nucleic acids [20]. Since their discovery in 1990, numerous aptamers have been identified for diverse targets, encompassing metal ions, organic molecules, peptide proteins, and whole cells [21]. In contrast to antibodies, aptamers exhibit sustained high binding affinities across a broad spectrum of conditions, coupled with advantageous attributes such as facile synthesis, versatile design capabilities, and enhanced stability. Nonetheless, conventional aptasensors often necessitate specialized personnel and expensive equipment and are primarily confined to laboratory settings, thereby constraining their applicability in point-of-need and point-of-care scenarios [17].
In recent years, lateral flow aptasensors (LFAs) have emerged as a promising avenue, eliciting escalating interest due to their cost-effectiveness and enhanced adaptability compared to antibody-based systems [23]. LFAs have been extensively explored for detecting various targets, spanning toxins, proteins, and cancer cells [4].
Advantages of aptamers over antibodies in lateral flow immunoassay
The LFIA is a widely used, simple, and cost-effective diagnostic method successfully applied in numerous medical tests, such as pregnancy tests and rapid infectious disease detection. By integrating aptamers into the LFIA format, we can create a rapid, specific, cost-effective D-dimer test suitable for use in low-resource and emergency settings. The proposed aptamer-based LFIA offers the potential for enhanced sensitivity, selectivity, and speed compared to existing antibody-based assays [22].
Therefore, this study aims to optimize an aptamer-based LFIA platform for the rapid detection of D-dimer in clinical samples, thereby offering a novel solution for the early detection of thrombotic disorders at the point of care. Combining the specificity of aptamers with the speed and ease of lateral flow technology could overcome the limitations of D-dimer testing, ultimately improving patient care through more timely and accurate diagnostics [4].
Antibodies may struggle to differentiate between closely related targets like small molecules, whereas aptamers can excel in this area. For example, Jenison et al. (1994) created an RNA aptamer that could distinguish between theophylline and caffeine despite their subtle chemical similarities. Unlike immunoassays, the aptamer showed a remarkable 10,000-fold preference for theophylline over caffeine [23].
In research conducted for the World Anti-Doping Agency (WADA), a series of DNA aptamers were engineered to be capable of discriminating between the virtually identical natural pituitary human growth hormone (hGH) and recombinant hGH produced in Escherichia coli bacteria- a feat challenging with antibodies. This discrimination was feasible due to alterations in up to 2% of recombinant hGH proteins induced by the bacterial host, validated by mass spectrometry [24, 25]. Similarly, the author successfully discriminated between isoleucine (I) and threonine (T) variants of prostate-specific antigen (PSA) at position 179, a task current antibodies struggle with, employing a diaminopurine (DAP)-modified aptamer and leveraging insights from a molecular docking model. The DAP-modified aptamer exhibited approximately a 20% disparity in colorimetric absorbance signals in an ELISA-like setting between the I- and T-PSA variants [25, 26].
An often overlooked yet significant distinction between aptamers and antibodies is their smaller size and weight than common IgG antibodies. Aptamers, typically comprising 70–200 bases, generally weigh approximately 20–60 kD, contrasting with IgG antibodies weighing over 150 kD. Moreover, the size of well-known aptamers, such as the thrombin aptamer, is notably smaller at approximately 21×25 Å compared to the substantially larger IgG antibodies at 122 × 139 Å [19].
Moreover, unlike antibodies, aptamers offer a unique detection modality involving hybridization or dehybridization of polymer strands, further augmenting their versatility and potential advantages in specific applications [17]. Herein, selected aptamers are derived through the SELEX process, single-stranded DNA or modified nucleic acids [27]. Numerous aptamers have been identified for diverse targets, encompassing metal ions, organic molecules, peptide proteins, and whole cells [28]. In contrast to antibodies, aptamers exhibit sustained high binding affinities across a broad spectrum of conditions, coupled with advantageous attributes such as facile synthesis, versatile design capabilities, and enhanced stability [18, 26]. Nonetheless, conventional aptasensors often necessitate specialized personnel and expensive equipment and are primarily confined to laboratory settings, thereby constraining their applicability in point-of-need and point-of-care scenarios [27].
In recent years, aptamer-based LFIAs (Fig. 2) (Table 1) have emerged as a promising avenue, eliciting escalating interest due to their cost-effectiveness and enhanced adaptability compared to antibody-based systems [29, 30].
LFAs in sensing technology
LFAs are a well-established sensing technology known for their rapid response, easy storage, user-friendly design, and on-site applicability without requiring instrumentation or additional chemicals. They can detect targets, including mycotoxins, small molecules, heavy metals, bacteria, viruses, and proteins [31].
LFAs utilize various detector reagents, from colloidal gold to dye-containing liposomes, and recent advancements include multiplex LFAs for the simultaneous detection of multiple targets [5]. Aptamer-based LFAs utilize aptamers' specificity, which is established during the selection process. They use reporter labels like gold nanoparticles, latex spheres, and quantum dots for assay specificity. LFAs offer simple, cost-effective detection of targets with high sensitivity [29]. In aptamer-based LFIAs, the sample with the analyte moves from the sample pad to the conjugate pad and binds to a labelled aptamer. The complex travels to the nitrocellulose membrane, where an immobilized aptamer captures it. Excess unbound complex is captured on the control line. Detection creates a signal on the test line and indicates proper liquid flow on the control line [30].

Fig. 2. Principles of aptamer-based lateral flow immunoassays (LFIAs). (A) The LFA strip includes four overlapping membranes: sample pad, conjugate pad, reaction membrane, and absorbent pad. (B)
LFIAs usage outside traditional laboratory settings, in point-of-care
One of the key advantages of LFIAs is their ability to provide immediate diagnosis and treatment during the same consultation, minimizing the need for multiple clinical visits, referrals, and delays in therapy initiation. For example, LFIAs can aid in distinguishing between bacterial and viral infections, helping clinicians decide whether antibiotic therapy is necessary and thereby mitigating the misuse of antibiotics, which contributes to the development of antibiotic resistance (Table 2). LFIA, like pregnancy tests, are widely used in clinical practice for various purposes due to their simplicity and quick results [29, 30]. LFIAs are particularly suitable for use outside traditional laboratory settings, including hospital wards, clinics, health centers, physicians' offices, and even in patients' homes for self-testing. This accessibility and ease of use make LFIAs valuable tools for point-of-care testing, enabling healthcare professionals to assess patients' conditions and make informed treatment decisions quickly [30] (Tables 3, 4).
AuNP-labeling of aptamer-based LFIA platforms
Gold nanoparticles (AuNPs) have become a widely adopted labeling system in lateral flow tests, attributed to their distinctive optical properties and straightforward conjugation with biomolecules [32]. The vibrant red hue of AuNPs, which arises from surface plasmon resonance, facilitates their detection in lateral flow assays. surface plasmon resonance occurs when the conduction electrons on the AuNP surface oscillate collectively in response to illumination, absorbing light in the blue-green spectrum while reflecting light in the red spectrum.
One of the key advantages of LFIAs is their ability to provide immediate diagnosis and treatment during the same consultation, minimizing the need for multiple clinical visits, referrals, and delays in therapy initiation. For example, LFIAs can aid in distinguishing between bacterial and viral infections, helping clinicians decide whether antibiotic therapy is necessary and thereby mitigating the misuse of antibiotics, which contributes to the development of antibiotic resistance (Table 2). LFIA, like pregnancy tests, are widely used in clinical practice for various purposes due to their simplicity and quick results [29, 30]. LFIAs are particularly suitable for use outside traditional laboratory settings, including hospital wards, clinics, health centers, physicians' offices, and even in patients' homes for self-testing. This accessibility and ease of use make LFIAs valuable tools for point-of-care testing, enabling healthcare professionals to assess patients' conditions and make informed treatment decisions quickly [30] (Tables 3, 4).
AuNP-labeling of aptamer-based LFIA platforms
Gold nanoparticles (AuNPs) have become a widely adopted labeling system in lateral flow tests, attributed to their distinctive optical properties and straightforward conjugation with biomolecules [32]. The vibrant red hue of AuNPs, which arises from surface plasmon resonance, facilitates their detection in lateral flow assays. surface plasmon resonance occurs when the conduction electrons on the AuNP surface oscillate collectively in response to illumination, absorbing light in the blue-green spectrum while reflecting light in the red spectrum.
Table 1. LFIAs usage outside traditional laboratory settings, its advantages and disadvantages [22, 30].
| Advantages | Disadvantages |
| Easy to use | Mainly qualitative or semi-quantitative. |
| Low-cost | Solid samples need to be Extracted. |
| Very fast turnaround time | Subjective result interpretation. |
| Lightweight and portable | Confirmatory analysis is needed (usually for positive results). |
| Limited sample volume required | Possible batch-to-batch variability |
| Limited sample treatment | Technological improvements usually increase the cost per analysis. |
| No need for instrumentation | - |
| Long storage stability (1-2 years) | - |
| Consolidated development process | - |
This property enhances the sensitivity and visibility of assays, making AuNPs an effective choice for various diagnostic applications [33]. AuNPs are versatile nanoparticles that can easily be combined with biomolecules through simple adsorption or gold-thiol surface chemistry. They are widely used in applications such as food analysis, diagnostics, and environmental monitoring, including lateral flow tests. Recent advances have allowed the development of nucleic acid detection tests using DNA probes and aptamers, which offer high specificity and affinity for target molecules. Innovative strategies like competitive assays and "reverse-aggregation" approaches have been created, with aptamers being split into two parts without losing activity for multiplex testing. This has enabled simultaneous detection of multiple targets in a logic-gate fashion. The use of aptamers in AuNP-based lateral flow tests has enhanced assay specificity and versatility, promising rapid and sensitive diagnostic applications (Table 3) [34]. Studies have employed an AuNP-based LFIA platform, integrating aptamers for specific D-dimer recognition. The assays have been optimized using clinical samples, with statistical analyses performed to determine sensitivity, specificity, and detection limits. A comparative analysis with ELISA was conducted to validate the assay's performance. The statistical significance has been assessed using receiver operating characteristic curve analysis, with a p-value< 0.05 considered significant [3, 35].
Different types of aptamer-based LFIA formats
Overall, aptamers offer versatile recognition elements for LFIAs, enabling the development of rapid, sensitive, and specific assays for a wide range of analytes across different categories. Their use in LFIAs holds promise for various applications in clinical diagnostics, environmental monitoring, and biomedical research. Over recent decades, several approaches for aptamer-based LFIAs have emerged, including sandwich formats, competitive formats, and innovative methods leveraging the structural and functional characteristics of aptamers [42] (Table 4).
Implementing dual aptamers in sandwich-type LFIAs is crucial for broader acceptance, as this approach enhances robustness through the use of excess labeled reporter aptamers and allows for the straightforward establishment of control lines. Moreover, the direct immobilization of capture aptamers onto membranes eliminates the need for protein storage, lowering costs and enabling non-refrigerated storage. Transitioning from competitive to sandwich formats also streamlines multiplexed aptamer detection, significantly improving assays' versatility.
The anticipated integration of dual aptamers and direct immobilization techniques across various membrane types is expected to promote the successful implementation and commercial adoption of aptamer-based LFIAs [43, 44].
Different types of aptamer-based LFIA formats
Overall, aptamers offer versatile recognition elements for LFIAs, enabling the development of rapid, sensitive, and specific assays for a wide range of analytes across different categories. Their use in LFIAs holds promise for various applications in clinical diagnostics, environmental monitoring, and biomedical research. Over recent decades, several approaches for aptamer-based LFIAs have emerged, including sandwich formats, competitive formats, and innovative methods leveraging the structural and functional characteristics of aptamers [42] (Table 4).
Implementing dual aptamers in sandwich-type LFIAs is crucial for broader acceptance, as this approach enhances robustness through the use of excess labeled reporter aptamers and allows for the straightforward establishment of control lines. Moreover, the direct immobilization of capture aptamers onto membranes eliminates the need for protein storage, lowering costs and enabling non-refrigerated storage. Transitioning from competitive to sandwich formats also streamlines multiplexed aptamer detection, significantly improving assays' versatility.
The anticipated integration of dual aptamers and direct immobilization techniques across various membrane types is expected to promote the successful implementation and commercial adoption of aptamer-based LFIAs [43, 44].
Table 2. Examples of aptamer-based lateral flow immunoassays (LFIA) using AuNPs labels in probes for the detection of biomarkers.
| Strategy | Biomarker | Label/ Ref. |
| Conjugation of AuNP's with dopamine duplex aptamer and dissociation of duplexes in the presence of the target | Dopamine | AuNPs [35] |
| Desorption of biotin-modified aptamer from AuNPs surface in the presence of analyte | HER2 | AuNPs [36] |
| The competitive reaction between CA125 conjugated with AuNPs and unlabeled CA125 for binding to capture probe. | CA125 | AuNPs Enzyme [34] |
| An aptamer-based hook-effect-recognizable three-line LFIA | Thrombin | AuNPs [37] |
| Binding of a biotinylated aptamer to target in the sample and reaction with AuNPs-streptavidin conjugate, then subsequent capturing by the antibody at the test line | Osteopontin | AuNPs [38] |
| A sandwich LFIA based on biotin-labeled primary aptamer immobilized on the streptavidin-coated membrane as a capturing probe and secondary aptamer conjugated with AuNPs as a signaling probe | Vaspin | AuNPs [16] |
Table 3. Examples of the applications of aptamers in lateral flow immunoassays (LFIAs) for various types of analytes
| 1-Cell-based detection | Aptamers can be used in LFIAs to detect specific cell types or cell surface markers. For example, aptamers selected against cancer cells or specific cell surface proteins can be immobilized on the test line of LFIAs to capture target cells present in the sample. The binding of target cells to aptamer-coated nanoparticles or surfaces results in a visible signal, allowing for rapid and sensitive cell detection [39]. |
| 2-Protein-based detection | Aptamers have been widely employed in LFIAs to detect proteins, including disease biomarkers, antigens, and toxins. In these assays, aptamers specific to the target protein are immobilized on the test line, while labeled aptamers or aptamer-functionalized nanoparticles are used as detection probes. Upon binding the target protein to the immobilized aptamer, a visible signal is generated, indicating the presence of the protein in the sample [40]. |
| 3-Small molecule-based detection | Aptamers can also be utilized in LFAs to detect small molecules such as drugs, metabolites, and environmental pollutants. In these assays, aptamers selected to bind to the target small molecule are immobilized on the test line, while labeled aptamers or aptamer-functionalized nanoparticles are used for detection. The binding of the target small molecule to the immobilized aptamer results in a visible signal, enabling rapid and specific detection [28]. |
| 4-Ionic substance detection | Aptamers with affinity for specific ions or ion-containing compounds can be employed in LFIAs to detect ionic substances. In these assays, aptamers capable of binding to the target ion are immobilized on the test line, while labeled aptamers or aptamer-functionalized nanoparticles are used for detection. The binding of the target ion to the immobilized aptamer leads to a visible signal, allowing for rapid and selective detection of the ionic substance [41]. |
Table 4. Existing aptamer-based LFIA formats, including sandwich and competitive formats [21, 45].
| Sandwich aptamer-based LFIAs (Apt-LFA) |
| 1. The sandwich assay method represents the predominant approach in Apt-LFA, particularly for detecting large molecular weight analytes such as proteins. In a typical sandwich Apt-LFA setup, the process begins with a sample application to the sample pad. Here, the target molecules are initially captured by a detection aptamer, which is often conjugated with a reporter molecule. This interaction leads to the formation of a complex between the reporter-conjugated aptamer and the target on the conjugate pad. |
| 2. As the sample migrates towards the test line, the target molecule within the complex encounters a secondary affinity agent immobilized at the test line. This secondary agent recognizes the target and forms a sandwich structure: the target molecule is positioned between the reporter-conjugated aptamer and the secondary affinity agent at the test line. This formation confirms the presence of the target analyte. |
| 3. Three main types of sandwich Apt-LFAs have been proposed and utilized to date, optimizing target molecules' capture and detection through distinct design and operational principles. |
| Competitive aptamer-based LFIAs |
|
| 2. Target Immobilization: In this approach, target molecules are immobilized on the test line. The aptamer, specific to the target, competes with the free target molecules present in the sample for binding to the immobilized targets on the test line. |
| 3. Oligonucleotide Immobilization: Alternatively, partially complementary oligonucleotides to the aptamer can be immobilized on the test line. These oligonucleotides compete with the aptamer to bind to the target molecules in the sample [46]. |
Conclusion
The thromboembolic disease represents a considerable health risk, characterized by abnormal blood clotting and resulting in elevated morbidity and mortality rates. Various assays have been developed to detect and monitor this condition, primarily using monoclonal antibodies that target specific epitopes on cross-linked D-dimer fragments. D-dimers, which are unique products of cross-linked fibrin degradation, serve as indicators of thromboembolic events. D-dimer levels are frequently assessed to assess thromboembolic disease in patients with complex medical conditions effectively. Central laboratories typically utilize automated testing technologies, such as ELISA, latex-enhanced immunoturbidimetry, and chemiluminescence assays. In contrast, clinics and urgent care facilities depend on advanced bedside testing methods, including immunochromatography assays, to enable swift D-dimer evaluation. As a result, healthcare providers and clinics must remain attentive to the specific D-dimer assay used in their facility to ensure optimal patient care in diagnosing and managing thromboembolic disease. In conclusion, the various aptamer-based LFIAs discussed above demonstrate their potential as cost-effective, sensitive, and portable methods for the rapid detection of a wide range of targets within 5 to 30 minutes, achieving limits of detection that rival those of traditional laboratory techniques. However, the commercialization of these assays has been limited, with only a few documented instances of market introduction. Despite this, the unique benefits of aptamers - such as lower production costs, increased stability, and ease of modification and immobilization - position aptamer-based LFIAs as strong alternatives to conventional lateral flow immunoassays. This trend will likely result in a marked increase in published studies and promote the successful implementation and commercial adoption of aptamer-based LFIAs.
Ethical Considerations
Ethical approval for this study was obtained from the Ethics Committee of Shahid Sadouqhi University of Medical Sciences under the approved number: IR.SSU.MEDICINE.REC.1403.008.
Funding
This research received no external funding.
Conflict of Interests
The authors declare that there is no conflict of interest.
Acknowledgements
Not applicable.
Authors’ Contributions
F.P, E.O: Conceptualization, E.O: Investigation and writing original draft, F.P: Writing, review & editing.
Ethical Considerations
Ethical approval for this study was obtained from the Ethics Committee of Shahid Sadouqhi University of Medical Sciences under the approved number: IR.SSU.MEDICINE.REC.1403.008.
Funding
This research received no external funding.
Conflict of Interests
The authors declare that there is no conflict of interest.
Acknowledgements
Not applicable.
Authors’ Contributions
F.P, E.O: Conceptualization, E.O: Investigation and writing original draft, F.P: Writing, review & editing.
References
- Favresse J, Lippi G, Roy PM, Chatelain B, Jacqmin H, Ten Cate H, et al. D-dimer: Preanalytical, analytical, postanalytical variables, and clinical applications. Thromb Res. 2018; 55(8): 548-77.
- Yang H, Ji J, Liu Y, Kong J, Liu B. An aptamer-based biosensor for sensitive thrombin detection. Biosens Bioelectron 2009; 11(1): 38-40.
- Ayass MA, Griko N, Lubag A, Abi Mosleh L. D-dimer-specific aptamers and methods of use in diagnostics, therapeutic and theranostic purposes. Google Patents; 2019.
- Jauset-Rubio M, El-Shahawi MS, Bashammakh AS, Alyoubi AO, Ciara KJ. Advances in aptamers-based lateral flow assays. Trends Anal Chem. 2017; 97: 385-98.
- Majdinasab M, Badea M, Marty JL. Aptamer-based lateral flow assays: Current trends in clinical diagnostic rapid tests. Biosensors 2022; 15(1): 90.
- Stang LJ. D-dimer and fibrinogen/fibrin degradation products. J Hematol Methods Protocols 2013: 415-27.
- Sadeghi-Nodoushan F, Zare-Khormizi MR, Hekmatimoghaddam S, Pourrajab F. Blood features associated with viral infection severity: An experience from COVID‐19‐pandemic patients hospitalized in the center of Iran, Yazd. Int J Clin Pract. 2024; 2024(1): 7484645.
- Reber G, Moerloose P. Standardization of D‐dimer testing. J Lab Hematol Thrombosis. 2013: 136-46.
- Lip GY, Lowe GD. Fibrin D-dimer: a useful clinical marker of thrombogenesis? Clin Sci. 1995; 89(3): 205-14.
- Adam SS, Key NS, Greenberg CS. D-dimer antigen: current concepts and prospects. Blood 2009; 113(13): 2878-887.
- Freund Y, Cohen-Aubart F, Bloom BJ. Acute pulmonary embolism: a review. JAMA 2022; 328(13): 1336-345.
- Tasić N, Paixao TR, Gonçalves LM. Biosensing of D-dimer, making the transition from the central hospital laboratory to bedside determination. Biosens Bioelectron 2020; 207: 120270.
- Thachil J, Lippi G, Favaloro EJ. D-dimer testing: laboratory aspects and current issues. J Hematol Methods Protocols. 2017:91-104.
- Johnson ED, Schell JC, Rodgers GM. The D‐dimer assay. Am J Hematol Oncol. 2019; 94(7): 833-39.
- Riley RS, Gilbert AR, Dalton JB, Pai S, McPherson RA. Widely used types and clinical applications of D-dimer assay. Lab Med. 2016; 47(2): 90-102.
- Raston NHA, Nguyen V-T, Gu MB. A new lateral flow strip assay (LFSA) using a pair of aptamers for the detection of Vaspin. Biosens Bioelectron 2017; 93: 21-5.
- Bruno JG. Applications in which aptamers are needed or wanted in diagnostics and therapeutics. Pharmaceutics 2022; 15(6): 693.
- Sefah K, Phillips JA, Xiong X, Meng L, Van Simaeys D, Chen H, et al. Nucleic acid aptamers for biosensors and bio-analytical applications. Anal Chim Acta 2009; 134(9): 1765-775.
- Lee JF, Stovall GM, Ellington AD. Aptamer therapeutics advance. Curr Opin Chem Biol. 2006; 10(3): 282-89.
- Smirnov I, Shafer RH. Effect of loop sequence and size on DNA aptamer stability. Biochemistry 2000; 39(6): 1462-468.
- Dreymann N, Sabrowski W, Danso J, Menger MM. Aptamer-based sandwich assay formats for detection and discrimination of human high- and low-molecular-weight uPA for cancer prognosis and diagnosis. Cancers 2022; 14(21): 5222.
- Koczula KM, Gallotta A. Lateral flow assays. Essays Biochem 2016; 60(1): 111-20.
- Jenison RD, Gill SC, Pardi A, Polisky B. High-resolution molecular discrimination by RNA. Science 1994; 263(5152): 1425-429.
- Bruno JG, Carrillo MP, Phillips T, Edge AJ. Discrimination of recombinant from natural human growth hormone using DNA aptamers. J Biotechnol. 2011; 22(1): 27.
- Hepner F, Cszasar E, Roitinger E, Lubec G. Mass spectrometrical analysis of recombinant human growth hormone (Genotropin®) reveals amino acid substitutions in 2% of the expressed protein. Proteomics 2005; 3: 1-12.
- Kalra P, Dhiman A, Cho WC, Bruno JG, Sharma TK. Simple methods and rational design for enhancing aptamer sensitivity and specificity. Front Immunol. 2018; 5: 41.
- Liu G, Gurung AS, Qiu W. Lateral flow aptasensor for simultaneous detection of platelet-derived growth factor-BB (PDGF-BB) and thrombin. Biosens Bioelectron 2019; 24(4): 756.
- Liu Y, Liu B, Xia L, Yu H, Wang Q, Wu Y, et al. Cationic polyelectrolyte as powerful capture molecule in aptamer-based chromatographic strip for rapid visual detection of paraquat residue in agricultural products. Food Chem. 2022; 368: 132237.
- Soh JH, Chan HM, Ying JY. Strategies for developing sensitive and specific nanoparticle-based lateral flow assays as point-of-care diagnostic device. Nanotechnology 2020; 30: 100831.
- Di Nardo F, Chiarello M, Cavalera S, Baggiani C, Anfossi L. Ten years of lateral flow immunoassay technique applications: Trends, challenges and future perspectives. Sensors 2021; 21(15): 5185.
- Bahadır EB, Sezgintürk MK. Lateral flow assays: Principles, designs and labels. Trends Anal Chem. 2016; 82: 286-306.
- Sadeghi Nodoushan F, Hakimian F, Haghiralsadat BF, Taghiyar S. The effect of superparamagnetic Fe3O4 nanoparticles combined with quercetin on breast cancer cell line (MCF-7). J Nanostruct. 2023; 8(4): 565-76.
- Glomm WR. Functionalized gold nanoparticles for applications in bionanotechnology. J Disp Sci Technol. 2005; 26(3): 389-414.
- Tripathi P, Kumar A, Sachan M, Gupta S, Nara S. Aptamer-gold nanozyme based competitive lateral flow assay for rapid detection of CA125 in human serum. Biosens Bioelectron. 2020; 165: 112368.
- Dalirirad S, Steckl AJ. Lateral flow assay using aptamer-based sensing for on-site detection of dopamine in urine. Anal Bioanal Chem. 2020; 596: 113637.
- Ranganathan V, Srinivasan S, Singh A, DeRosa MC. An aptamer-based colorimetric lateral flow assay for the detection of human epidermal growth factor receptor 2 (HER2). Anal Biochem. 2020; 588: 113471.
- Gao Y, Zhu Z, Xi X, Cao T, Wen W, Zhang X, et al. An aptamer-based hook-effect-recognizable three-line lateral flow biosensor for rapid detection of thrombin. Biosens Bioelectron. 2019; 133: 177-82.
- Mukama O, Wu W, Wu J, Lu X, Liu Y, Liu Y, et al. A highly sensitive and specific lateral flow aptasensor for the detection of human osteopontin. Biosens Bioelectron. 2020; 210: 120624.
- Kiplagat A, Martin DR, Onani MO, Meyer MJ. Aptamer-conjugated magnetic nanoparticles for the efficient capture of cancer biomarker proteins. J Mater Sci Mater Med. 2020; 497: 166063.
- Li G, Li Q, Wang X, Liu X, Zhang Y, Li R, et al. Lateral flow immunoassays for antigens, antibodies and haptens detection. Biosens Bioelectron. 2023; 242: 125186.
- Wu Z, He D, Xu E, Jiao A, Chughtai MF, Jin Z. Rapid detection of β-conglutin with a novel lateral flow aptasensor assisted by immunomagnetic enrichment and enzyme signal amplification. Food Chem. 2018; 269: 375-79.
- Liang G, Cai S, Zhang P, Peng Y, Chen H, Zhang S, et al. Magnetic relaxation switch and colorimetric detection of thrombin using aptamer-functionalized gold-coated iron oxide nanoparticles. Biosens Bioelectron 2011; 689(2): 243-49.
- Seo HB, Gu MB. Aptamer-based sandwich-type biosensors. Biosens Bioelectron. 2017; 11: 1-7.
- Schüling T, Eilers A, Scheper T, Walter J. Aptamer-based lateral flow assays. Anal Bioanal Chem. 2018; 5(2): 78-102.
- Jin B, Yang Y, He R, Park YI, Lee A, Bai D, et al. Lateral flow aptamer assay integrated smartphone-based portable device for simultaneous detection of multiple targets using upconversion nanoparticles. Biosens Bioelectron. 2018; 276: 48-56.
- Wang T, Chen L, Chikkanna A, Chen S, Brusius I, Sbuh N, et al. Development of nucleic acid aptamer-based lateral flow assays: A robust platform for cost-effective point-of-care diagnosis. Biosens Bioelectron. 2021; 11(11): 5174.
Type of Study: Research |
Subject:
Biochemistry
Received: 2024/12/30 | Accepted: 2025/04/29 | Published: 2024/10/1
Received: 2024/12/30 | Accepted: 2025/04/29 | Published: 2024/10/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






